The humoral immune response involves antibody-mediated specific immunity. This type of immune response is transferable to unimmunized recipients by utilizing immune serum containing the specific antibody of the pathogen. (Janeway et al., 2005)
Although the cellular mediated response plays the main role in fighting infection through the activation of T cell defense systems (including the release of numerous cytokines), antibodies are still utilized as part of the immune response to the invading R. rickettsii bacteria. These conclusions can be drawn based on the fact that humans who become infected with the bacteria become immune to further infection. Laboratory tests have shown that in wild-type and immunocompromised SCID mice, antibodies are able to control the infection enough so as to prevent both types of mice from lethal infections. Additional research has investigated the concept of cross-reactive immune responses involving R.rickettsii, which involves the binding of an antibody to an antigen that was not used to elicit that antibody. One such study showed that the antigens of Rickettsia rhipicephali, a nonpathogenic spotted fever group of Rickettsia, were partially protected against the challenge by pathogenic rickettsiae like R. rickettsii. Although there is not much known about this cross-protective response, this field of research holds the potential for the possible identification and characterization of similar antigens between nonpathogenic and pathogenic species that could lead to information about rickettsial immunity. (Gage et al., 1992)
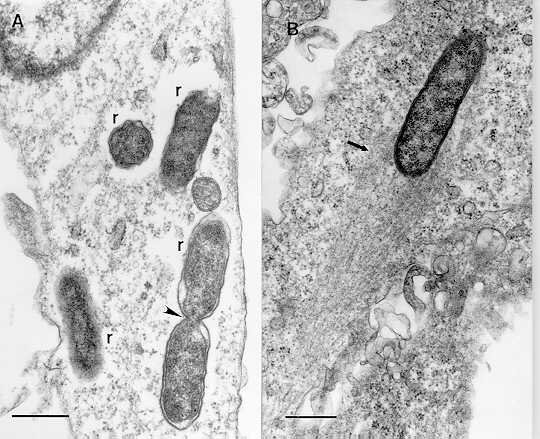
Organisms of Rickettsia conorii (r), a close relative of R. rickettsii, in a cultured human endothelial cell are located free in the cytosol. One rickettsia is dividing by binary fission (arrowhead). (B) These rickettsiae can move inside the cytoplasm of the host cell because of the propulsive force created by the "tail" of host cell actin filaments (arrow). Bars = 0.5 µm.
Photo and text courtesy of David H. Walker - http://gsbs.utmb.edu/microbook/ch038.htm
There is also additional evidence that supports the secondary importance of humoral immunity just behind cellular immunity. Studies have shown that passive transfer of immune sera is not sufficient to protect animals from rickettsial infections. It does, however, often reduce the severity of the disease and aid the cellular immune response in clearing the pathogen from the host cell. The antibodies produced by rickettsiae do have some cell-mediated immune response capabilities, however. Pretreatment of rickettsiae with antibodies neutralizes infections in animals. Therefore, in these laboratory experiments, the antibodies to these surface epitopes can play an important effector role. Unfortunately, it is not known whether the same effect would occur in vivo. (Gage et al., 1992)
The humoral response begins at the bacterial outer membrane proteins (OMPs). OMP antibodies (especially IgG2a) have very high binding affinities and long half-lives throughout binding activity. The antibodies require Fc (constant region) receptors in order for them to function properly. These antibodies have the ability to eliminate the bacteria from tissues within 24 hours after the infection. In this study by Winslow, Yager, and Shu-Yi Li of an alternative rickettsial bacterium (Ehrlichia chaffeensis) did not show any significant roles for the complement cascade, reactive nitrogen species, neutrophils, or NK cells. (Winslow et al., 2003)
The antibody to epitopes of OmpA and OmpB contribute specifically to the immune protection in Rickettsia rickettsii. Group cross-protective immune protection requires the joint activation of all mechanisms. (Walker et al., 2003) There are a few different possible mechanisms for the activation of the humoral immune response. First, antibodies or other immune complexes trigger microbiodial activities in macrophages that result in the elimination of the bacteria within them. Second, it is possible that antibodies opsonize bacteria exposed during intercellular transfer. (Winslow et al., 2003)